TRANSKINTM is a cutting edge artificial composite and skin model that is sourced from (South Asia) human tissue, engineered and reconstructed in vitro. TRANSKINTM is composed of upper epithelial layers, followed by metabolically active stroma with stellate cells. Arterial and venous connections are structured in the stromal section mimicking multi-layer tissue construct.
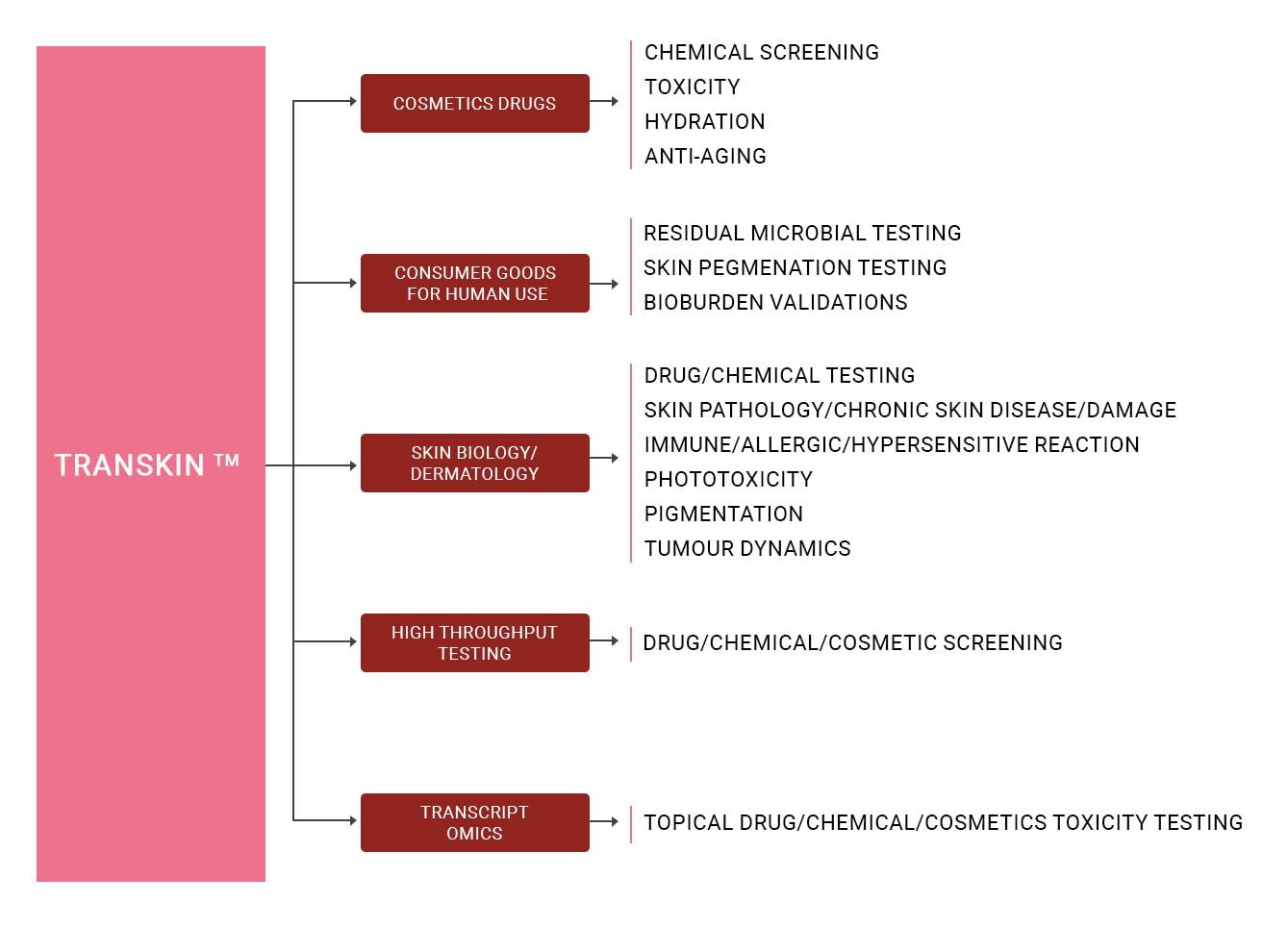
The artificial skin model is relevant to preclinical in vitro applications, including, but not limited to, aspects of dermatology, screening of drug candidates, cosmetics, and other chemicals (excipients) intended for human application. It is suitable also for studies of diseases like Psoriasis, Ichthyosis, eczema, of burn wounds, or of chronic skin wounds such as those caused by diabetes. TRANSKINTM can be also be customized for several applications, for instance, for the study of allergic/ hypersensitivity reactions and of skin microbiota. In all these applications and others, the model is predictive of the biology of human skin.
TRANSKINTM is suitable as a platform skin model to perform transcriptomics profile in response to the test material added. Chemicals elicit (signature) RNA profiles that are sufficiently distinct for their safety classing, and transcriptomic analyses surpassing qPCR-based analyses in throughput and accuracy.
TRANSKINTM can also be provided in a format suitable/ compatible with HTS, to expedite the rapid testing of a chemical library, and for any further downstream analyses.
TRANSKINTM is produced according to a simple, robust, and proprietary procedure to exacting technological standards, and suffers from minimal batch-to-batch variations. It is customized to the customer’s specifications with scale. All relevant details of the tissue’s provenance, collection, processing to produce TRANSKINTM, storage, and distribution to customers upon request.
With a commitment in advancing in vitro solutions and services to support customers’ research and validate their product claims, TRANSKINTM endorses animal-free testing for drugs and cosmetics through it’s features and supports the ban on animal testing for all chemicals in the EU, India, and several countries worldwide. TRANSKINTM is compliant with all Indian and US FDA regulations, and with all EU regulations governing the import and use of human tissues and cells for the in vitro purposes while the user customizes the assay methods and protocols depending on the test to be performed.




Reviews
There are no reviews yet.